Part:BBa_K4040013
mIL-6R
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21COMPATIBLE WITH RFC[21]
- 23COMPATIBLE WITH RFC[23]
- 25INCOMPATIBLE WITH RFC[25]Illegal NgoMIV site found at 142
Illegal NgoMIV site found at 166
Illegal NgoMIV site found at 238
Illegal NgoMIV site found at 250 - 1000COMPATIBLE WITH RFC[1000]
Usage and Biology
This gene encodes a subunit of the interleukin 6 (IL6) receptor complex that binds to IL6 with low affinity, but does not transduce a signal[1]. The IL6 receptor is a protein complex consisting of this protein and interleukin 6 signal transducer (GP130BBa_K4040032), a receptor subunit also shared by many other cytokines. The classical signaling pathway is triggered by the hemerer complex formed by mIL-6R.
Background and detail description
IL-6/IL-6R SYSTEM
IL-6 exerts its biological activities through two molecules:IL-6R (also known as IL-6Rα, gp80 or CD126) and gp130 (also referred to as IL-6Rβ or CD130) [2]. IL-6R is important for ligand binding, but it only has 82 amino acids in its cytoplasmic domain, indicating that it can play only a minor role in signal transduction.However, the cytoplasmic tail of the IL-6R may play a decisive role in basolateral sorting, which is an important function in polarized epithelial cells [3]. In contrast, the cytoplasmic domain of gp130 contains several potential motifs for intracellular signalling, such as the YSTV sequence for SHP-2 (Src homology domain-containing protein tyrosine phosphatase-2) recruitment and YXXQ motifs (where X is any amino acid) for STAT (signal transducer and activator of transcription) activation. gp130 does not have an intrinsic kinase domain; instead, like other cytokine receptors, the cytoplasmic domain of gp130 contains regions required for its association with a non-receptor tyrosine kinase called JAK (Janus kinase), through which downstream signalling cascades are initiated.
When IL-6 binds to mIL-6R (membrane-bound IL-6R), homodimerization of gp130 is induced, and a high-affinity functional receptor complex of IL-6, IL-6R and gp130 is formed. sIL-6R (soluble IL-6R), which lacks the intracytoplasmic portion of mIL-6R and is produced either by the enzymatic cleavage of mIL-6R by ADAM (a disintegrin and metalloproteinase)-17 or by alternative splicing, can also bind with IL-6, and then the complex of IL-6 and sIL-6R can form a complex with gp130(Figure 1). This unique receptor signalling system is termed IL-6 trans-signalling [4].
The structure of the gp130–IL-6R–IL-6 complex has been solved by X-ray crystallography: it is a hexamer comprising two IL-6, two IL-6R and two gp130 proteins[5].It has been argued, however, that the signalling complex is built of one IL-6–IL-6R complex bound to two gp130 proteins [6].These two signals independently induce a variety of biological activities.
IL-6 SIGNAL TRANSDUCTION
It is thought that receptor homodimerization brings the JAKs into close proximity, resulting in their mutual transactivation. The activated JAKs phosphorylate tyrosine residues in the cytoplasmic domain of gp130. There are six tyrosine residues in the human gp130 cytoplasmic domain. The gp130-mediated JAK activation by IL-6 triggers two main signalling pathways: the gp130 Tyr759-derived SHP-2/ERK (extracellular-signal-regulated kinase) MAPK (mitogen-activated protein kinase) pathway and the gp130 YXXQ-mediated JAK/STAT pathway (Figure 2) [7].
In the gp130 Tyr759-derived SHP-2/ERK MAPK pathway, upon IL-6 stimulation, SHP-2 is recruited to the phosphorylated Tyr759residue of gp130. After being recruited, SHP-2 is phosphorylated by JAKs and then interacts with Grb2 (growth-factor-receptor-bound protein 2), which is constitutively associated with Sos(son-of-sevenless), a GDT/GTP exchanger for Ras. The GTP form of Ras transmits signals that lead to activation of the ERK MAPK cascade, which activates transcription factors such as NF-IL-6 [C/EBPβ (CCAAT/enhancer-binding proteinβ)] that can act through their own cognate response elements in the genome.In the gp130 YXXQ-mediated JAK/STAT pathway, upon IL-6 stimulation, STAT proteins are recruited to the phosphorylated YXXQ/YXPQ motifs and are then phosphorylated by JAKs. The activated STAT proteins form a heterodimer (STAT1–STAT3) or homodimers (STAT1–STAT1 and/or STAT3–STAT3), subsequently translocate to the nucleus and activate the transcription of target genes. Interestingly, SOCS (suppressor of cytokine signalling) is one of the target genes of the JAK/STAT pathway. SOCS inhibits JAK activity and thus negatively regulates the signals [8], suggesting the existence of an autoregulatory mechanism for this signalling pathway.
These two signals independently induce a variety of biological activities. For example, we have reported that the SHP-2/ERK MAPK pathway induces MMP (matrix metalloproteinase) production and the JAK/STAT pathway induces RANKL (receptor activator of nuclear factor κB ligand) expression in synovial cells [9,10].
USED FOR A SYNTHETIC RECEPTOR
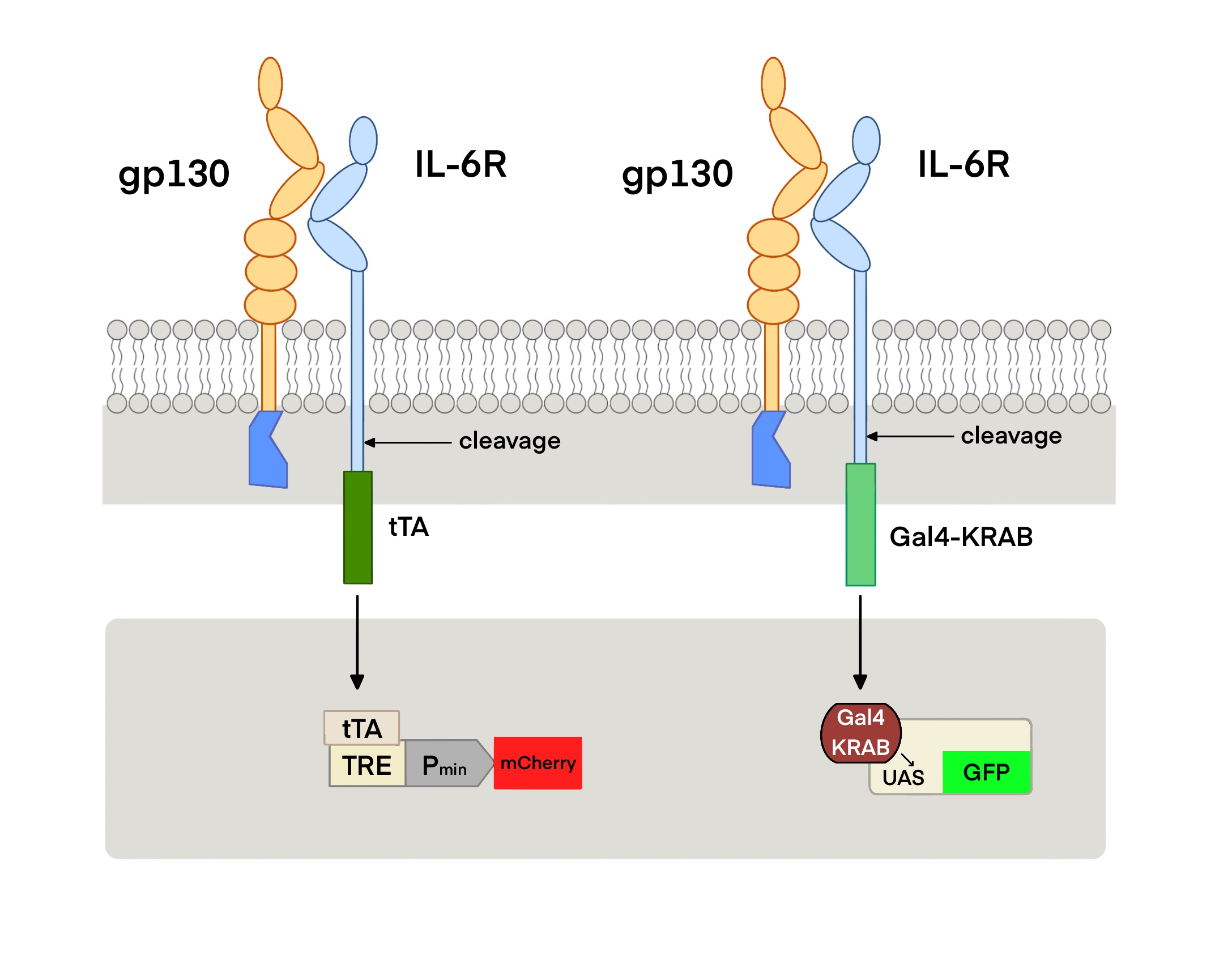
We designed the IL-6 cytokine sensor based on the Tango/MESA concept. Tango is an artificial receptor for detecting soluble molecules. The receptor consists of three parts: ① Binding transcription factors at the cytoplasmic C-terminal of a natural receptor. A specific restriction site is inserted between the receptor and the transcription factor, which forms a fusion protein with receptor – restriction site - transcription factor; ② In the activated state, the receptor recruits proteins and those proteins connect to proteases that can perform the enzyme digestion function at the restriction site; ③ Reporter genes and other target genes activated by transcription factors.
Because IL-6 binds to IL-6 receptor (IL-6R) and glycoprotein 130 (GP130) to form a hexamer complex, the IL-6 cytokine sensor in our project is composed of two parts: one is composed of transmembrane protein GP130 and tobacco etch virus (TEV) protease. The other is composed of the extracellular segment of IL-6R, TEV cleavage site (TCS) and transcription factors of tobacco etch virus. When the concentration of IL-6 in the body increases to a certain level, the hexamer complex formed activates TEV protease, causing the release of transcription factors, which triggers the subsequent pathway.
More information of the usage can be found in IL6R-TCS-Gal4-KRAB(BBa_K4040021) and IL6R-TCS-tTA(BBa_K4040022).
References
[1]Larsen JV, Petersen CM. SorLA in Interleukin-6 Signaling and Turnover. Mol Cell Biol. 2017 May 16;37(11):e00641-16. doi: 10.1128/MCB.00641-16. PMID: 28265003; PMCID: PMC5440653.
[2]Hibi, M., Murakami, M., Saito, M., Hirano, T., Taga, T. and Kishimoto, T. (1990) Molecular cloning and expression of an IL-6 signal transducer, gp130. Cell 63, 1149–1157
[3]Martens, A. S., Bode, J. G., Heinrich, P . C. and Graeve, L.(2000) The cytoplasmic domain of the interleukin-6 receptor gp80 mediates its basolateral sorting in polarized Madin–Darby canine kidney cells. J. Cell Sci. 113, 3593–3602
[4]Rose-John, S. and Neurath, M. F . (2004) IL-6 trans-signaling: the heat is on. Immunity 20, 2–4
[5]Boulanger, M. J., Chow, D. C., Brevnova, E. E. and Garcia, K. C. (2003) Hexameric structure and assembly of the interleukin-6/IL-6α receptor/gp130 complex. Science 300, 2101–2104
[6]Grötzinger, J., Kernebeck, T., Kallen, K. J. and Rose-John, S. (1999) IL-6 type cytokine receptor complexes: hexamer or tetramer or both? Biol. Chem. 380, 803–813
[7]Hirano, T., Nakajima, K. and Hibi, M. (1997) Signaling mechanisms through gp130: a model of the cytokine system. Cytokine Growth Factor Rev. 8, 241–252
[8]Starr, R. and Hilton, D. J. (1998) SOCS: suppressors of cytokine signalling. Int. J. Biochem. Cell Biol. 30, 1081–1085
[9]Hashizume, M. and Mihara, M. (2010) High molecular weight hyaluronic acid inhibits IL-6-induced MMP production from human chondrocytes by up-regulating the ERK inhibitor, MKP-1. Biochem. Biophys. Res. Commun. 403, 184–189
[10]Hashizume, M., Hayakawa, N. and Mihara, M. (2008) IL-6 trans-signalling directly induces RANKL on fibroblast-like synovial cells and is involved in RANKL induction by TNF-α and IL-17. Rheumatology 47, 1635–1640
| None |